Manufacturer-Centric Traceability Systems
Developing effective national traceability systems requires far more than regulatory mandates or government enforcement: it demands scalable digital traceability solutions that address real supply chain challenges and the operational realities of manufacturers.
A track and trace system that fails to align with manufacturers’ production workflows, serialization and aggregation processes, or data management constraints may technically meet regulatory compliance standards, but it will struggle in real-world implementation.
To build reliable, future-ready traceability infrastructure, governments and stakeholders must design systems around the actual needs of manufacturers, the first and most critical link in the traceability chain.
Manufacturers are the ones applying unique identifiers, managing data integrity, and generating the foundational product information that fuels supply chain visibility, in the pharmaceutical sector, for example, from production to patient. Placing them at the core of traceability system design ensures smoother integration, operational efficiency, and greater compliance success across the supply chain.
The Risk of Designing in Isolation
Overlooking Operational Complexity
If we consider the pharmaceutical traceability system design, ignoring the operational complexity of real manufacturing environments is one of the most common, and costly, mistakes.
The operations of pharmaceutical production lines vary significantly between companies depending on their size, product portfolio, and export markets. While regional supply facilities may run fully automated serialization and aggregation lines, smaller plants often handle niche batch production with manual or semi-automated setups.
When traceability vendors make assumptions about uniform production models, they end up developing generic and static track and trace solutions that fail during real-world implementation.
Vendors who lack practical understanding of serialization implementation, OEE (Overall Equipment Effectiveness) impact, printer-MES system connectivity, or production schedule management risk creating technology that obstructs pharma manufacturers rather than enabling regulatory compliance and operational efficiency.
Data Volume and Format Misunderstandings
Accurate serialization data management is central to every pharmaceutical traceability system. This data does not exist in isolation, it flows across multiple systems such as ERP, WMS, Level 3 site servers, and aggregation layers before reaching the corporate-level repository or national traceability database.
A lack of understanding of actual data structures, validation protocols, and storage practices often results in a system-data mismatch.
If a track and trace solution expects data formats or flows that don’t align with the manufacturer’s existing equipment architecture or IT infrastructure, the burden of adjustment falls entirely on the manufacturer, leading to production delays, non-compliance risks, and resistance to adoption.
Strong data integrity and system interoperability must therefore be foundational design principles for any pharma serialization platform.
Imposing Unrealistic Timelines or System Loads
Another common pitfall in pharma traceability implementation is underestimating the importance of system performance testing.
Solutions that lack staging environments, retry mechanisms, or robust validation frameworks often collapse when real-time production data begins to flow.
When vendors fail to grasp how serial number generation, aggregation logic, and data upload to national systems operate under actual factory conditions, they overlook common performance challenges, turning their platforms into production bottlenecks rather than enablers of compliance and traceability.
Ignoring these operational realities forces manufacturers to develop temporary manual workarounds, increasing the risk of data inconsistencies, serialization errors, and non-compliant packaging records. This not only threatens regulatory approval but can also compromise pharmaceutical supply chain integrity and weaken the entire traceability framework.
Designing National Pharma Traceability Systems
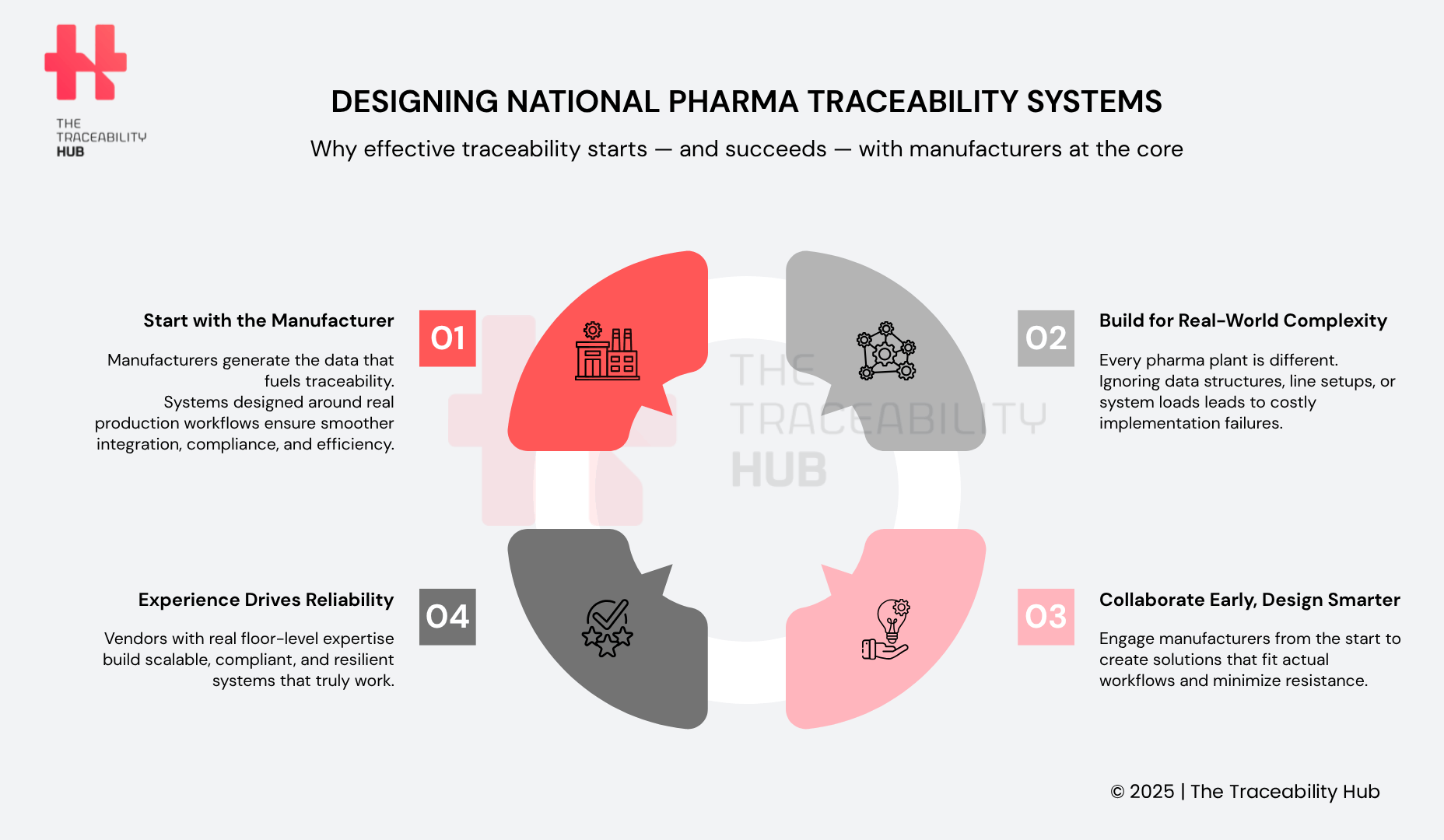
Why Listening to Manufacturers Matters
Designing for Real Use, Not Ideal Scenarios
In the development of pharmaceutical traceability systems, the most successful track and trace implementations are those built with direct manufacturer engagement from the very beginning.
Vendors who initiate early collaboration with pharmaceutical manufacturers gain critical insights into production cycles, serialization technology choices, and regulatory compliance requirements.
This real-world understanding ensures that platform development aligns with actual manufacturing operations, minimizing rework, reducing downtime, and improving system adoption rates across production sites.
Digital traceability solutions designed for real use, not just theoretical models, deliver smoother onboarding experiences, higher data interoperability, and more dependable operational performance within pharma manufacturing environments.
Lower Resistance, Higher Compliance
Early and meaningful manufacturer participation in the design of national traceability systems leads to faster system deployment and more sustainable compliance outcomes.
Solutions that respect manufacturers’ operational limitations while meeting traceability and serialization regulations result in:
- Higher traceability compliance rates
- Fewer technical integration issues
- Stronger regulatory collaboration between authorities and manufacturers
By creating manufacturer-friendly traceability platforms, governments and technology vendors can build trust, simplify data exchange, and accelerate the implementation of compliant track and trace systems.
Reliable Data Starts with Manufacturer-Friendly Tools
The data integrity of any traceability system depends entirely on the quality of serialization data generated by manufacturers.
If traceability platforms fail to integrate properly with existing manufacturing execution systems (MES) or lack robust data validation mechanisms, the accuracy and reliability of the entire pharma traceability network deteriorate.
By aligning government traceability frameworks with actual manufacturing processes and operational realities, authorities can ensure:
- Consistent traceability data quality
- Faster, smoother system rollouts
- Stronger supply chain visibility and regulatory transparency
This alignment creates the foundation for interruption-free national traceability implementation, enabling secure, transparent, and compliant pharmaceutical supply chains.
Case for Experience: Not All Vendors Understand the Floor
In pharmaceutical traceability system design, not all traceability vendors truly understand the realities of manufacturing floor operations.
A vendor with proven serialization implementation experience across multiple pharma production lines and countries delivers far more reliable and compliant track and trace solutions.
Experienced traceability technology providers understand the complex workflows of pharmaceutical manufacturing, from production scheduling to MES connectivity and data integrity management.
Their hands-on knowledge allows them to anticipate potential production bottlenecks and resolve issues before they interrupt manufacturing operations or regulatory reporting.
Why Experience Matters in Traceability Implementation
Vendors with extensive pharma serialization experience bring field-tested insights that directly improve system performance, compliance reliability, and supply chain visibility.
This expertise leads to tangible technical advantages:
- Pre-configured data formats that match Level-3 (L3) system expectations, reducing integration time and ensuring data consistency across the traceability ecosystem.
- API interfaces validated under real production loads, supporting seamless data exchange between MES, ERP, and national traceability databases.
- User-friendly system dashboards focused on what manufacturers care about, serialization success rates, pending uploads, and exception log monitoring.
These capabilities help manufacturers achieve regulatory compliance, maintain production efficiency, and strengthen overall traceability system performance.
By choosing a traceability vendor with real-world manufacturing floor experience, companies ensure that their track and trace solutions are not only compliant but also resilient, scalable, and optimized for continuous digital transformation within the pharmaceutical supply chain.
A Better National System Starts with the Right Questions
The success of national pharmaceutical traceability systems depends on how well governments understand the readiness of manufacturers to implement serialization and aggregation across their production environments.
Before launching a national track and trace platform, authorities must evaluate manufacturers’ technical capacity, infrastructure maturity, and ability to integrate seamlessly with traceability compliance systems.
Key questions every traceability implementation authority should ask include:
- How many manufacturers are ready to integrate with the national traceability platform using their current serialization technology?
- What support systems and technical helpdesks are available to manage reporting challenges or traceability data transmission errors from manufacturers?
- How does the system handle partial or failed transactions to prevent data integrity issues and ensure regulatory reporting accuracy?
- Can manufacturers operate offline temporarily without risking traceability data loss or non-compliance during connectivity disruptions?
These are not theoretical questions—they define the difference between a traceability system that functions reliably and one that collapses under real-world pressure.
The Right System Is the One That Works for Everyone
A successful national traceability system must support all stakeholders across the pharmaceutical supply chain, starting with the manufacturers, the first and most critical link in the serialization and aggregation process.
No system can achieve end-to-end traceability if manufacturers cannot participate effectively or if integration barriers slow adoption.
Governments should select traceability technology partners who not only understand regulatory frameworks but also possess deep knowledge of factory floor operations, MES connectivity, and coding line integration.
Real pharmaceutical track and trace systems are not built in isolation.
They emerge through public-private collaboration, continuous feedback loops, and active learning from the people who operate machines, code printers, and manage daily distribution within the pharma supply chain.
Developing functional national traceability ecosystems requires a practical, inclusive approach that prioritizes usability, scalability, and regulatory compliance, turning policy objectives into real-world success instead of theoretical performance.
Read more: FDA’s 12-Digit NDC Format: Essential Guide for Drug Supply Chain Teams